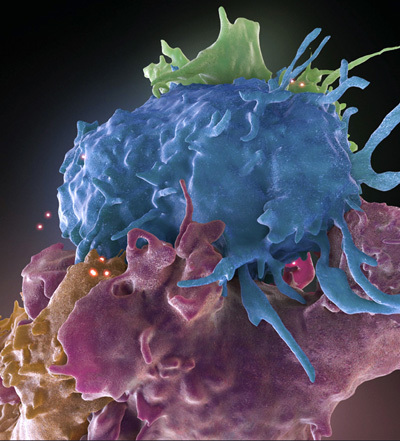
In the process of engineering CAR T cells, if the patient’s T cells are out of the body for too long, they can lose their ability to replicate, which is critical to their effectiveness as a live drug. Thus, a research team has developed a new approach that reduces the time needed to modify patients’ immune cells so that they can be quickly reinjected into the body. The manufacturing process for CAR T cells typically takes between nine and 14 days, whereas this new process would reduce the manufacturing time to as little as 24 hours. Traditional manufacturing approaches require that T cells be stimulated in a way that encourages the cells to replicate and grow in number. The key to the Penn researchers’ manufacturing approach is the lentiviral vector that delivers the CAR gene to T cells. Lentiviral vectors are able to transfer genes like the CAR gene to cells without needing this initial “activation” step. This approach has the dual advantage of speeding up the overall manufacturing process while maintaining T-cell potency. The team hopes that the reduced manufacturing time could make the therapy more cost-effective and accessible to more patients.