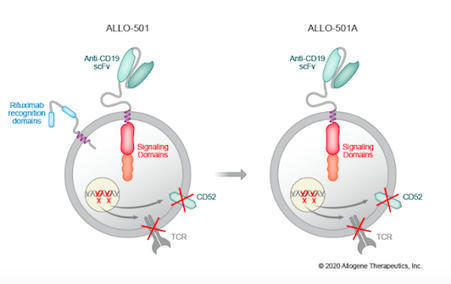
ALLO-501A is a CAR T cell-based therapy for relapsed or refractory large B-cell lymphoma developed by the American company Allogene Therapeutics. The advantage of this therapy is its allogeneic approach, i.e. it is based on healthy donor cells. However, the risk of graft-versus-host disease (GVHD) must be mitigated to avoid rejection of the therapy. The CD52 gene was therefore disrupted using Cellectis TALEN technology which is common in allogeneic CAR T therapies in order to have a long-term therapeutic effect. ALLO-501A was developed from a previous ALLO-501 therapy which has rituximab, an
anti-CD20 monoclonal antibody, recognition domains, whereas these are eliminated in ALLO-501A. This approach should allow for broader use of the therapy. ALLO-501A has been in a phase 1/2 trial, which is called Alpha-2, since June 2020 and has enrolled 120 participants. The goal of this trial is to evaluate safety of escalating doses, efficacy and cell kinetics in patients. Initial clinical data are expected to be shared at the 2021 American Society of Clinical Oncology annual meeting in June.